

Sang Wook Park
Associate Professor (Plant Stress Responses)
Entomology & Plant Pathology
(334) 844-1958
sww0011@auburn.edu
Get In Touch
Address:
228 Rouse Life Science Building
Auburn Univ, AL 36849
Biography
EDUCATION
- 2008: Postdoc., Boyce Thompson Institute for Plant Research, Cornell Univ.
- 2004: Ph.D., Plant Biochemistry, Colorado State Univ.
- 2000: M.S., Plant Biochemistry, Chung-Ang Univ., Korea
WORK EXPERIENCE
- Current: Assoc. Professor, Plant Pathology, Auburn Univ.
- 2021: Asst. Professor, Plant Pathology, Auburn Univ.
- 2014: Sr. Rsch. Asst., Virginia Bioinformatics Institute, Virginia Tech
Research
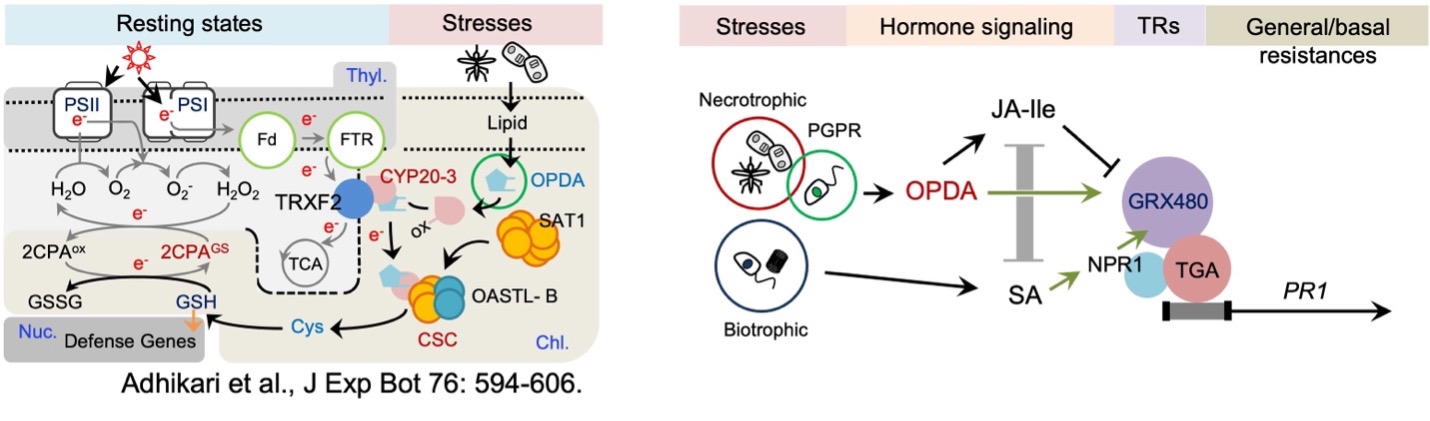
Modus operandi of phytodefense hormone 12-oxophytodienoic acid (OPDA) in plant disease resistances: OPDA binds and reduces a receptor, cyclophilin (CYP)20-3, by stimulating its interaction with thioredoxin (TRX)F2 that delivers electrons (e−) from the photosystem (PS)I. The activated CYP20-3 then splits serine acetyltransferase (SAT)1 (dimeric trimers) in half to bring about the recruitment of O-acetylserine(thiol)lyase (OASTL)-B dimer, forming a hetero-oligomeric cysteine (Cys) synthase complex (CSC) that assimilates sulfurs and generates Cys and glutathione (GSH), which in turn coordinates (i) the S-glutathionylation (activation) of 2-Cys peroxiredoxinA (2CPA, a recycler in the water-water cycle) in peroxide detoxification, while (ii) triggering the retrograde regulation of the expression of defense OPDA-responsive genes in the nucleus. This regulatory interface between growth and defense responses shapes the optimal growth plasticity and survival potential of plants under various environmental pressures. Chl, chloroplasts; Fd, ferredoxin; FTR, ferredoxin-thioredoxin reductase; 2CPAGS, S-glutathionylated 2CPA; Nuc, nucleous; ox, oxidized; TCA, tricarboxylic acid cycle; Thyl, thylakoid.
Engineering of for-profit drought tolerant crops: The cost of defense, referred to as the growth and defense tradeoffs, appears to be a major pitfall in the process of genetically engineering/improving defense capacity in plants (PMPP 95:55). In an effort to search for feasible approaches to enhance defense responses, many studies including ours have highlighted plant growth-promoting rhizobacteria (PGPR)-mediated Induced Systemic Tolerance (IST), unique mechanisms capable of priming drought and/or abiotic stress tolerance without growth. A caveat is that PGPR applications often display little reproducibility in the field, because a variety of endo/exogenous factors cause them to be unable to colonize plant roots (Biotechnol Lett 32: 1559). Thus, we have identified two PGPR-responsive genes, RD29s, that play critical roles in equipping plants with IST.

Publications
- Kaur S†, Adhikari A†, Thapa P, Liu W, Dobrev PI, Vaculiková R, Lacek J, Park SW (2025) A circadian clock RD29A is an esterase, relaying PGPR stimuli via RD29B and OPDA signaling in priming plant drought tolerance. Plant J. In press.
- Adhikari A, Kaur S, Forouhar F, Kale S, Park SW (2025) OPDA signaling channels resource (e-) allocation from the photosynthetic electron transfer chain to plastid cysteine biosynthesis in defense activation. J. Exp. Bot. 76: 594-606.
- Kaur S, Adhikari A, Welsh B, Gosse HN, Dos Santos IB, Liu W, Lawrence KS, Park SW (2025) Cyclophilin 20-3 A coordinates plant root hair growth and resistance against parasitic nematodes. Plant Sci. 354: 112432.
- Liu W, Thapa P, Park SW (2023) RD29A and RD29B rearrange genetic and epigenetic markers in priming systemic defense responses against drought and salinity. Plant Sci. 337: 111895.
- Adhikari A, Park SW (2023) Reduced GSH acts as a metabolic cue of OPDA signaling in coregulating photosynthesis and defense activation under stress. Plants 12: 3745.
- Gattoni KM, Park SW, Lawrence KS (2023) Evaluation of the mechanism of action of Bacillus spp. to manage Meloidogyne incognita with split root assay, RT-qPCR and qPCR. Front. Plant Sci. 13: 1079109.
- Liu W, Park SW (2021) 12-oxo-phytodienoic acid: a fuse and/or switch of plant growth and defense responses? Front. Plant Sci. 12: 724079.
- Liu W, Sikora E, Park SW (2020) Plant growth-promoting rhizobacterium, Paenibacillus polymyxa CR1, upregulates dehydration-responsive genes, RD29A and RD29B, during priming drought tolerance in Arabidopsis. Plant Physiol. Biochem. 156: 146-154.
- Liu W, Dos Santos IB, Moye A, Park SW (2020) CYP20-3 deglutathionylates 2-CysPRX A and suppresses peroxide detoxification during heat stress. Life Sci Alliance 3: e202000775.
- Subedi P, Gattoni K, Liu W, Lawrence KS, Park SW (2020) Current utility of plant growth-promoting rhizobacteria as biological control agents towards plant-parasitic nematodes. Plants 9: 1167.
- Dos Santos IB, Park SW (2019) Versatility of cyclophilins in plant growth and survival: a case study in Arabidopsis. Biomolecules 9: 20.
- Liu W, Jones AL, Gosse HN, Lawrence KS, Park SW (2019) Validation of the chemotaxis of plant parasitic nematodes toward host root exudates. J. Nematol. 51: e2019-63.
- Liu W, Park SW (2018) Underground mystery: interactions between plant roots and parasitic nematodes. Curr. Plant Biol. 15: 25-29.
- Cheong H, Dos Santos IB, Liu W, Gosse HN, Park SW (2017) Cyclophilin 20-3 is positioned as a regulatory hub between light-dependent redox and 12-oxo-phytodienoic acid signaling. Plant Signal. Behav. 12: e1362520
